Hormonal manipulation is likely one of the primary ways to hold PCa in check.
Short descriptions and list of abbreviations:
- ADT: Androgen Deprivation Therapy (reduction of androgens to very low levels: typically serum DHT and testosterone will be close to undetectable).
- AI: Aromatase Inhibitor. Some portion of testosterone is converted to estrogen via the aromatase enzyme. An aromatase inhibitor blocks this conversion.
- AR: Androgen Receptor
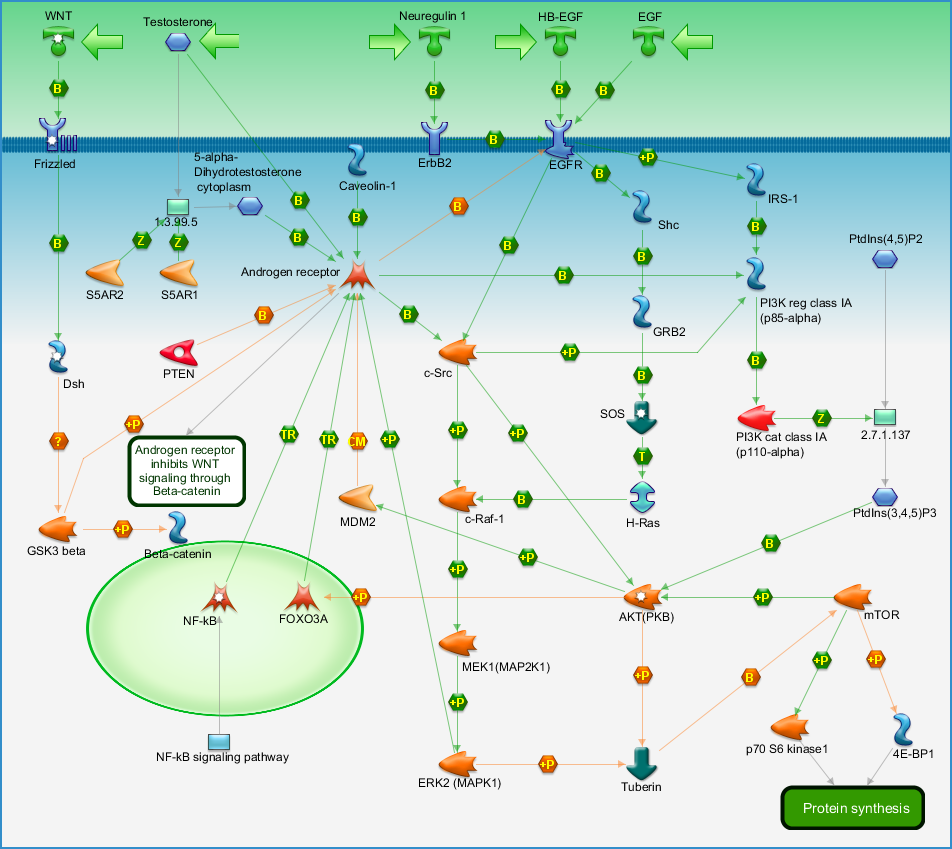
- mAR: membrane Androgen Receptor (outside of cell). Androgens binding to mAR might be beneficial.
- iAR: intracellular Androgen Receptor (inside of cell). Androgens binding to iAR might be detrimental.
- BAT: Bipolar Androgen Therapy. Testosterone is cycled from a high level (typically > 1500 ng/dl) to a low level (less than 50 ng/dl).
- BP: Blood Pressure
- CRPC: Castrate Resistant Prostate Cancer (essentially PCa that is not sensitive to a low testosterone environment).
- LT: Low serum total Testosterone (<50 ng/dl)
- MO: Medical Oncologist
- NT: Normal serum total Testosterone (around 200-1000 ng/dl)
- PCa: Prostate Cancer
- PSA: Prostate Specific Antigen (a marker that is often used to determine cancer progression. Testosterone can stimulate an increase that is not necessarily an indication of increased cancer activity. In addition, PSA is not 100% prostate specific. PSA is also produced in small amounts by other body tissues).
- SOC: Standard Of Care (FDA approved medical treatments)
- SPT: Supraphysiological Testosterone (>1500 ng/dl)
In the mid 20th century Dr. Charles Huggins researched how androgen levels affect PCa growth (https://cancerres.aacrjournals.org/content/canres/25/7_Part_1/1163.full.pdf). He came up with two main conclusions.
A) Low levels of testosterone led to the death or weakening of some PCa cells.
B) High levels of testosterone led to the death or weakening of some PCa cells.
Unfortunately for us the main takeaway that conventional doctors pursued was conclusion A.
Huggins’ research and subsequent research has given us the following insights:
- At low testosterone levels the easiest to control PCa cells are stopped and / or killed. Progression of these cells is halted the most in a low testosterone environment. Thus SOC use of ADT to try to get testosterone to low testosterone levels. This typically is successful for a year or two, after which the surviving cells begin mutating to castrate resistant prostate cancer and begin accelerated rapid growth. Another theory is that a very small number of cells aren’t dependent on androgens to survive. These cells continue to proliferate in the low androgen environment (ADT). As they proliferate and the androgen dependent cells die off, the androgen independent cells take over and eventually become a new, more dangerous, threat. What we do know is that we typically end up with castrate resistant prostate cancer.
- Castrate resistant prostate cancer is a much more aggressive form of cancer and is very hard to control since it evolves in a low testosterone or normal testosterone environment. Even while working, ADT has extremely negative side effects. Without testosterone depression is likely, it is almost guaranteed that one will lose muscle mass and muscle mass is very necessary for quality of life and is even more important as we become older. Libido will likely go to zero. Bone loss will occur to many of the men on ADT. Estrogen based ADT will alleviate the depression and bone loss. But not the libido or muscle wasting. It works in part by inhibiting luteinizing hormone. This hormone stimulates leydig cells to produce testosterone in the testes.
- In a normal testosterone environment all PCa cells are able to grow.
- At supraphysiological testosterone levels cancer cells are affected negatively. Huggins thought that PCa was one of the exceptions to the rule. He formed this conclusion because one individual did not respond to high testosterone.
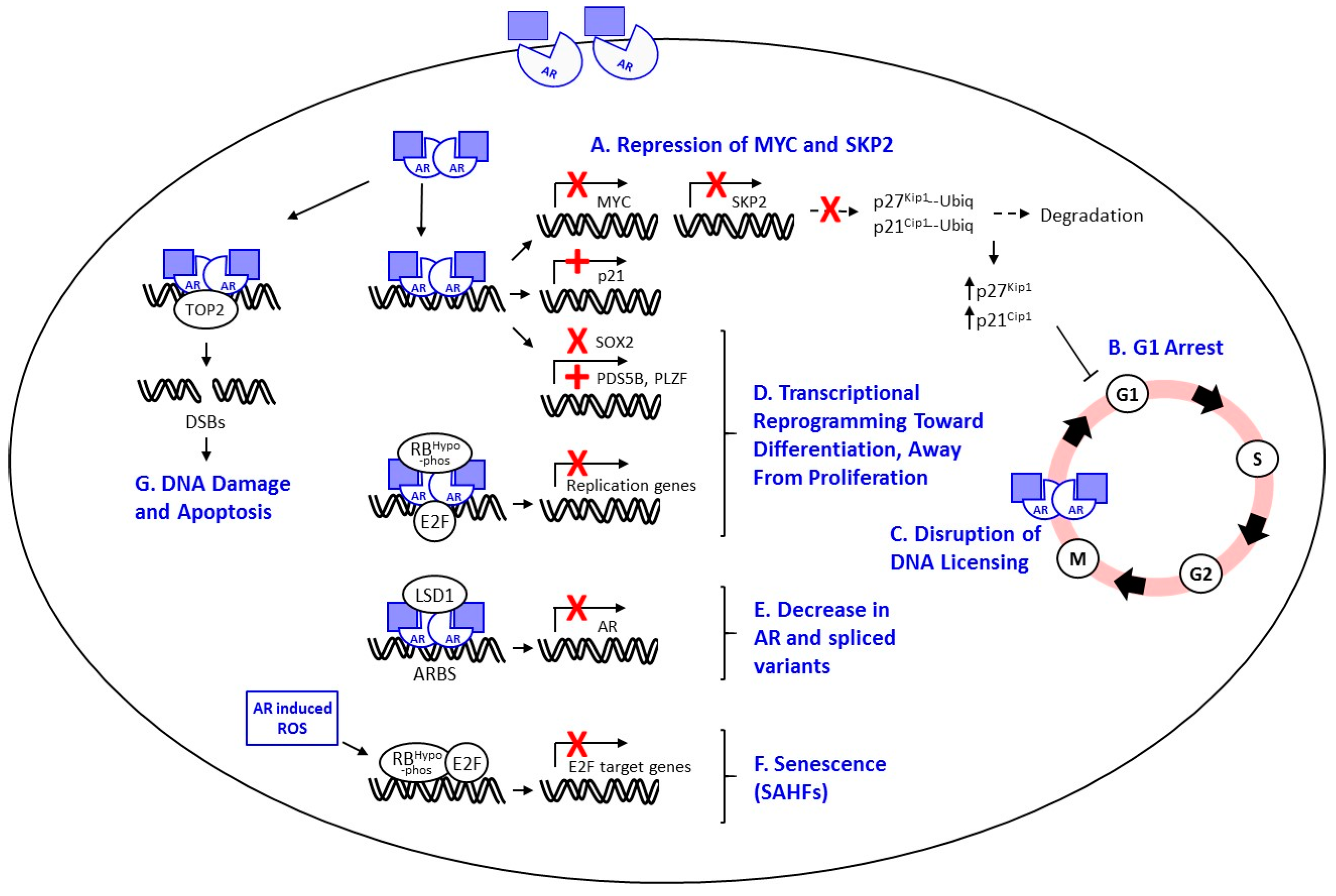
I’m not sure if supraphysiological testosterone works via double-strand breaks in the DNA, calcium ion influx (a little is bad, a lot kills PCa and supraphysiological testosterone supposedly pushes a lot of calcium ions into the cancer cells), or something else. Another theory is that the cancer cells become bloated with testosterone. Then the cells must expel all testosterone in order to start the next licensing process to begin a new doubling (dividing) cycle. So, as long as supraphysiologic testosterone is maintained, they are dormant or at the very worst, slow growing. It has also been theorized that at supraphysiologic testosterone levels one will not become castrate resistant.
Even if there is slow rise in PSA it usually doesn’t matter. If your PSA doubling time on supraphysiologic testosterone is (lets say) 18 months it would take 30 years for a low volume of disease to grow enough to kill you. And it is very possible that within 30 years we will have other more successful treatments for Pca.
Bipolar Androgen Therapy (BAT)
Johns Hopkins and others have come up with a technique that they call BAT.
To perform BAT you cycle testosterone between low levels and supraphysiological levels. BAT is typically used for hormone insensitive prostate cancer to attempt to restore some hormone sensitivity to it. There are many studies and clinical trials for this procedure. I’m hormone sensitive (earlier stage) but recently a clinical trial used BAT for hormone sensitive prostate cancer and the results were good for some men. Researchers at Johns Hopkins are also studying the early application of BAT. The theory is that you might be able to put off or completely eliminate the hormone insensitive phase (this phase occurs when PCa only requires a little testosterone to live). When PCa becomes hormone insensitive most people die in 12 – 27 months. Note that this is using standard of care – but some people are going strong decades after ADT and many of them are using BAT to restore hormone sensitivity. We’re not sure why some people react this well to ADT and/or BAT while others don’t. It might be because of certain gene variants in the cancer and researchers are looking into this.
Clinical trials and studies have used time-frames from 2 weeks to 6 months for the low testosterone phase.
During the supraphysiological testosterone phase I think it will be important to use an aromatase inhibitor in addition to testosterone. The aromatase inhibitor will further increase testosterone and decrease estrogen. It does this by blocking the testosterone to estrogen conversion.
Many researchers use some form of ADT during the supraphysiological testosterone phase. ADT will take your body’s production of testosterone low but with the exogenous testosterone injections your overall testosterone will be high. In this manner, when you go off of the exogenous testosterone your testosterone should go to zero rapidly (depending on when your last injection was the exogenous testosterone will leave your system in 0-4 weeks – testosterone cypionate has a 6 day half-life). Another possibility is to switch to bioidentical testosterone cream or gels at the end of a “high” cycle and prior to going “low”. If you do that the upside is that the testosterone leaves your body in a matter of days instead of weeks. However, I haven’t come across any research using this method for exogenous testosterone and creams and gels might be insufficient to achieve supraphysiological testosterone levels.
Since I have been using exogenous testosterone for over a year my body’s endogenous production of testosterone should be zero. However, if my body’s endogenous testosterone production isn’t zero I might use estrogen patches to reduce my testosterone (see estrogen post for details). I don’t want high testosterone and high estrogen at the same time so I’ll come off of estrogen patches as soon as I start the supraphysiological testosterone phase and resume at the end (I might adjust this a little). I hope to get to zero testosterone quickly. I also take Zytiga. Zytiga operates by halting the production of testosterone by the adrenal glands (and prostate cells). I plan to continue Zytiga throughout all cycles. There is research showing that it drops testosterone very low by itself. I don’t understand this unless you’ve had an orchiectomy.
The overall duration of the BAT protocol is dynamic and depends on how PSA is moving and PCa is progressing. I haven’t decided when to stop BAT. It probably will depend on my PSA levels and cancer scans. Of particular interest to me is a newer scan called PSMA-PET-CT scan. Research indicates that it is more effective than a typical scan and the radiation is considerably reduced. It works by scanning for a protein that is overexpressed by prostate cancer cells (Prostate Specific Membrane Antigen). However, when this was written, the PSMA-PET scans were not approved in the U.S. and therefore not covered by insurance.

I recently read a study about Zytiga vs. conventional ADT. Apparently Zytiga/Prednisone alone might be able to take the place of ADT. Results from this study indicate that estrogen patches might not be necessary even if my endogenous testosterone doesn’t go to zero.
HORMONES:
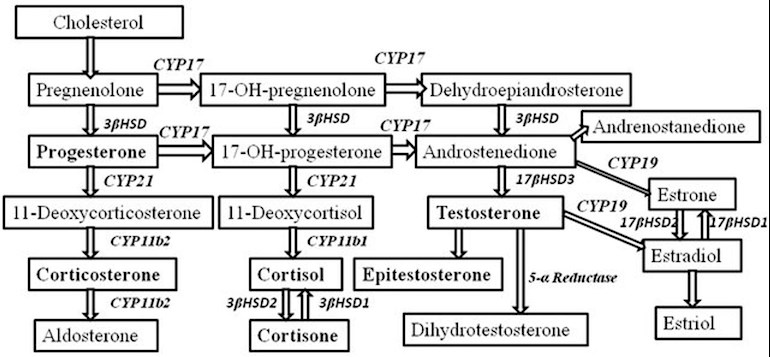
- DHEA: DHEA is sometimes called the mother hormone. It converts into androgens, estrogen, and progesterone, and also activates estrogen receptor alpha (procancer) and estrogen receptor beta (possibly anti-cancer). DHEA keeps cortisol in check. Cortisol is necessary in small amounts to control stress. But in large amounts (which is typical in our stressed society) it can be a negative and prematurely age you. 7-keto-DHEA is a metabolite of DHEA and has very weak conversion to androgens and its primary interaction with estrogen receptors is with estrogen receptor beta. 7-keto-DHEA might speed up metabolism and heat production, resulting in fat loss. It also might improve lean body mass and build muscle and also boost the immune system and increase the activity of the thyroid gland. DHEA typically decreases in men as they age.
- DHT: Dihydrotestosterone. Typically 5-10% of your testosterone is converted to DHT. DHT translocates androgen receptors from the membrane to intracellular locations. DHT is an androgen that has about 5 times the androgenic activity as testosterone. DHT has anti-estrogenic effects. It is possibly procancer and possibly anti-cancer. DHT typically decreases in men as they age.
- Estrogen: There are 3 types of estrogen. Estrone (E1), estradiol (E2), and estriol (E3). E1 preferentially binds to estrogen receptor alpha and is procancer. Estradiol binds to estrogen receptor alpha and also to beta. On balance it is procancer. Estriol binds preferentially to estrogen receptor beta. It might be anti-cancer, neutral, or procancer since beta receptor activity is both procancer and anti-cancer. Estrogens typically increase in men as they age.
- HCG: Human chorionic gonadotropin. This hormone acts in a similar manner as luteinizing hormone and stimulates sperm production in the testes. Depending on your hormonal makeup it can also increase testosterone. It typically increases as you age.
- Growth Hormone: Growth hormone is procancer. Some evidence shows that it reduces lifespan. It typically decreases with age.
- Insulin: A hormone released by the pancreas that regulates blood sugar. Some of its actions are procancer. But I don’t think that we should try to directly control it. However it makes sense to naturally reduce insulin spikes and overall levels. If you eat fruit then by eating the fruit with some protein, some fat, and some soluble fiber (e.g. a few grams of guar gum), blood sugar spikes should be reduced and therefore insulin spikes are also reduced.
- Nitric Oxide: This is not a hormone. I include it though because it is a pro-aging free radical induced by estrogen and it promotes growth hormone. At one time I was try to increase my nitric oxide for exercise purposes. After further research I stopped.
- Pregnenolone: A precursor to many other hormones, including corticosteroids. A portion of it converts to DHEA and thus it might be procancer. It typically decreases as you get older.
- Progesterone: This hormone counters the effects of estrogens. It typically decreases as you get older.
- Prolactin: A hormone released by the pituitary gland. It stimulates some release of testosterone, is synergistic with estrogen and is procancer. Prolactin is similar in some of its actions to growth hormone. It typically increases as you get older. As a side note, when I decreased prolactin I experienced substantial and rapid fat loss (from around 13% to around 10%).
- Prostaglandins: Unlike most hormones, prostaglandins are not produced by glands but rather at the location that they are needed. Prostaglandins are part of the body’s response to stress and injury. They help create inflammation and are also potentially procancer. Aspirin reduces prostaglandin production (via COX-1 and COX-2 inhibition). Naproxen also reduces prostaglandins through similar mechanisms. Curcumin selectively inhibits COX-2 and might be a safer way to reduce prostaglandins than aspirin or naproxen. However, the substance has not been studied as extensively as aspirin or naproxen. It is also not as tightly regulated. Prostaglandin reduction can be good but must not be overdone. Some inflammation is necessary to heal wounds.
- SHBG is a protein made by your liver. It binds tightly to 3 sex hormones found in both men and women. These hormones are estrogen, dihydrotestosterone (DHT), and testosterone. SHBG carries these 3 hormones throughout your blood. SHBG decreases free and bioavailable testosterone. The decrease is proportionate to the levels of SHBG. If SHBG is high then even if serum total testosterone is high, the available testosterone might be low. I was a case in point. My total testosterone was about 1000 ng/dl prior to PCa. My SHBG, however, was extremely high (300 nmol/l) so my free and bioavailable testosterone were both low. In effect, I was hypogonadal. Something has changed since I have been on high testosterone and my SHBG has decreased to less than 100. In general SHBG increases with age and this exacerbates the effects of decreasing testosterone.
- Testosterone: A hormone primarily produced by the testes. It is androgenic and anabolic. Therefore it is responsible for male sex characteristics and also for muscle mass and sex drive. It is also important for bone mass. A feedback loop reduces LH and FSH as testosterone increases (of note for men who desire children: LH and FSH stimulate the testes to produce sperm). The reduction normally serves to keep testosterone levels in a desired range since LH causes testosterone to increase. A portion of testosterone is converted into DHT and a portion is converted into estrogen.
This is my BAT plan:
I used estrogen ADT to go low testosterone for 5 months. I added Zytiga but I’m not sure if it was necessary. Then I was on casodex and dutasteride for 1 month. My total testosterone was 2400 ng/dl. But since my androgen receptors were mostly blocked, the testosterone wasn’t active and therefore for practical purposes this extended my low testosterone phase to 6 months.
High phase (2+ months – as of 02/2021 I’ve been on this approximately 16 months). This will be continued until PSA and cancer progression is above some threshold (TBD) and/or PSA velocity or absolute level are above some thresholds (TBD) :
- Testosterone cypionate injections 400 mg/wk.
- Zytiga (1000 mg w/o food or possibly lower w/ food).
- aromatase inhibitor (keep estradiol around 15-25 pg/ml).
- Cabergoline (.25-.5 mg/wk to keep prolactin low).
- Dutasteride (.5 mg daily) and/or finasteride (2.5-5 mg daily) to keep DHT moderately low (perhaps 25-50 ng/dl). Duta and fina are 5-alpha reductase inhibitors and thus block the conversion of testosterone to DHT.
- I plan to cycle dutasteride and finasteride. Duta has a long half-life and fina has a short one. So my cycling plan is the following:
- Months 1-4: Duta every day and fina every day.
- Month 5: Nothing
- Month 6: Fina every day (no duta).
- Month 7: Nothing
- Month 8 Fina every day (no duta).
- Month 9: Nothing (Duta will have washed out of my system, fina has a short half life and will have washed out also, and therefore DHT will not be blocked during month 9).
- Repeat from step 1.
- I might change this. I might take duta for Months 1-2 and then fina on months 3-4. The other months would remain unchanged. This should eliminate some side effects from taking two different meds at a time and also allow duta to wash out of my system before month 5. This would let me to see how much DHT blocking fina does and how much duta does (duta has harsher side effects yet supposedly reduces DHT by 90% while fina only reduces it by 70%). It also would lend itself to a DHT cycling program.
- I plan to cycle dutasteride and finasteride. Duta has a long half-life and fina has a short one. So my cycling plan is the following:
- 7-keto-DHEA 100mg a day.
- Aspirin 81 mg/day.
- Naproxen 200 mg – 7 days a month in the morning.
- Curcumin/turmeric: I take a few grams of a curcumin/turmeric mix each day. Supposedly reduces prostaglandins and therefore inflammation. Supposedly a selective COX-2 inhibitor. Supplements aren’t well regulated and therefore the quality and activity is more variable than aspirin, etc.
- If PSA increases above 0.04:
- Supraphysiological testosterone without dutasteride and finasteride. Follow a vegan Mediterranean diet. Add PEMF 90+ minutes a day (PEMF: pulsed electromagnetic frequency). IR sauna a few hours a week (IR: infrared).
- I exceeded this on 11/24/2020 so I am following the outlined program. PSA at the start = 0.043 ng/ml. PSA at the end = 0.056 ng/ml.
2. If PSA increases above 0.05:
- Same as above but change to a Keto diet. Could be coincidence but each time my PSA has risen I have done 1-2 weeks of Keto and my PSA has dropped each time (twice from 0.06->0 and once from 0.06>0.028).
- I exceeded this on 12/19/2021 so on the majority of days I following a strict Keto diet. My PSA at the start was 0.056 ng/ml and at the end it was 0.059 ng/ml (03/04/2021).
- Update: 03/04/2021: Rather than wait for another possible PSA increase I added finasteride (a DHT blocker) back into my program. I also ate all of my food in a 1 hour window. I went on Keto for 2 week. On most days I drank an average of 4.6 mg of molecular hydrogen from water and I also inhaled some molecular hydrogen gas. In a little under one month (from 03/04/2021 to 03/30/2021) my PSA dropped from 0.059 ng/ml to 0.027 ng/ml.
3. If PSA increases above 0.065:
- Same as above but without 7-keto-DHEA and with a plant based mediterranean diet and a once a week 24+ hour fast. Other options are to let DHT float or take it low and to use a pregnenolone cream.
5. If PSA increases above 0.08:
- Same as above but consume 2.4-4.8 mg/day from hydrogen water.
6. If PSA increases above 0.09:
- Same as above but add in a 4 day fast each month.
7. If PSA increases above 0.1:
- Same as above but add finasteride back.
8. If PSA increases above 0.11:
- Same as above but stop blocking prolactin.
9. High phase but if PSA increases above 0.12: I’ll talk to my SOC MO and try other therapies (TBD) and possibly radiation (you can tell that I will do almost anything to stay high T – feel like a 20 year old and I don’t want to go back to “normal” even for a month).
If my PSA is less than 0.03 I’m going to try dropping Zytiga for a few months. I don’t know what it is adding to the supraphysiological testosterone. It might be increasing my BP (my MO thinks it’s possible and I did a short experiment and the results point to a BP increase from Zytiga). And, if I don’t need Zytiga right now I don’t want to have my cancer be desensitized to it.
Low phase (.5-1.5 months) if PSA > TBD and/or PSA doubling time < TBD:
- Zytiga (1000 mg w/o food or possibly lower w/ food).
- Cabergoline (.25-.5 mg/wk to keep prolactin low).
- Possibly add a calcium channel blocker. High testosterone works, in part, by sending a high amount of ca2 ions into cancer cells via voltage-gated calcium channels. Ca2 influx at low levels furthers PCa growth. At high levels it promotes apoptosis (cell death). So, with the testosterone reduced to low levels, calcium influx should be reduced. A calcium channel blocker such as Verapamil, Diltiazem, Nifedipine, or Amlodipine can be added. This needs careful thought.
- Possibly add Flutamide. Flutamide blocks the androgen receptors and has a short half-life (7 hours). It might interact with Zytiga though (Zytiga has a half-life of about 12 hours and should be sufficient by itself). I haven’t looked into this. Hopefully it will not be necessary.
- Possibly estrogen patches to reduce testosterone. If these are needed I’ll have to think about the timing. I hesitate to use estrogen since it is procancer. However, without testosterone the procancer effects should be decreased.
- Possibly add in dutasteride and/or finasteride. DHT should be close to zero since T is zero. If it isn’t then DHT blockers might be of use.
I’ll monitor the following metrics:
- Testosterone: during SPT I want this to be >1500 ng/dl. During ADT I want this to be <20 ng/dl.
- Free testosterone: during SPT I want this to be > 15 ng/dl.
- Bioavailable testosterone: during SPT I want this to be > 100 ng/dl.
- Prolactin: during SPT I want this to be < 5 ng/ml.
- DHEA: 175 to 300 ug/dl ?
- DHT: I don’t know if this should be low or high or normal. Normal would require use of a 5-ARI (dutasteride and/or finasteride) since 5-10% of testosterone is converted to DHT. If your testosterone is 3000 ng/dl that means that your DHT would be 150-300 (high).
- CEA: If this goes above 5 it indicates possible cancer growth. It isn’t a reliable test for PCa though and some variation is to be expected. It is more useful for trend monitoring.
- PSA (I would expect higher levels during supraphysiological testosterone therapy because testosterone should cause cancer cells and tissue cells to produce more PSA, regardless of whether PCa is growing or not).
- PSA velocity. Look at the rate of change of PSA level.
- NLR = neutrophils/lymphocytes: should be less than 2.
- dNLR = neutrophils/(WBC-neutrophils): should be less than 1.4.
- PNI = 10*Albumin (g/dl) + 5*Lymphocytes (absolute count * 10^3/L): should be > 50.
- PLR = platelets/lymphocytes. should be <100.
- HsCRP should be < 1.
- Fibrinogen: should be less than .5.
- Calcium (part of CMP test): Should be less than 10.5.
- Sodium (part of CMP test): should be between 135 and 145.
- Carbon Diox (part of CMP test): should be between 20 and 38.
- AST (part of CMP test): liver enzyme should be less than 38.
- ALT (part of CMP test): liver enzyme should be less than 45.
- ALP (part of CMP test): liver enzyme should be less than 125.
- Hematocrit: important to monitor during SPT, should be between 32% and 50%. If it goes too high then giving blood should lower it.
- Estrogen (estradiol is generally used as a proxy). I might measure estriol and estrone every once in a while. I normally use a standard low resolution test for estradiol but sometimes use a highly sensitive estradiol test (more expensive so I only do this if I think I need it). During SPT I want estradiol between 20 pg/ml and 30 pg/ml.
I’ve already verified testosterone levels and subjective effects from my high phase program. Serum T levels 2000-3000+ ng/dl. Good libido, good muscle gain, good fat loss, excellent energy and mood. It doesn’t seem like Zytiga is taking away from the supraphysiological testosterone.
Since supraphysiological testosterone causes DNA damage (double strand breaks) to the PCa cells and effectively kills them or keeps them in check it is possible that the supraphysiological testosterone phase will last indefinitely. Verification of this should fall out from the monitoring during the high phase. If this theory is correct then the low phase will never be entered.
My personal story of ADT and supraphysiological testosterone. I did ADT with estrogen patches to enter a low testosterone environment. It was very successful from that regard and, for the most part, my testosterone was undetectable. I didn’t suffer from any depression. But my muscles wasted away until all but my essential muscles were gone. My libido was zero. I also developed gyno (man boobs). I went through this for 5 months and then switched to a casodex/dutasteride combination. This combo allowed my testosterone to go high yet blocked the androgen receptors so that most of the testosterone was unused. I wasn’t on this long enough to determine its effects on QoL but I doubt that they would be substantially different than ADT. After the month was over I went on supraphysiological testosterone. Libido came back, muscle came back and then some, fat has gone down, energy has been higher than it has been in decades, minor injuries heal in days rather than months or years, I can work out hard and be ready to get into the gym again later in the day. I use an aromatase inhibitor since it is important to keep estradiol low during supraphysiological testosterone therapy and I no longer have any gyno. I feel incredible and some of my friends without PCa have since gone on supraphysiological testosterone therapy or TRT (lower dose testosterone replacement therapy).
Knowing what I now know and how I feel I would go on TRT even if I did not have PCa. But, because I have PCa, I don’t feel that TRT is beneficial and I think that I need to continue supraphysiological testosterone.
Side effects of supraphysiological testosterone:
- Testosterone inhibits luteinizing hormone. Luteinizing hormone stimulates the testes to produce sperm. Therefore male sperm count decreases during supraphysiological testosterone therapy. I’m older and not interested in having more kids so this isn’t an issue (plus I had my prostate surgically removed so it’s moot anyway).
- HCG might act as a stimulant in place of luteinizing hormone. However, I am not sure how HCG reacts with PCa. I’ve only seen a few studies and the slant seems to be that HCG might be a bad idea for PCa.
- Testosterone increases red blood cell production. This can cause hematocrit to increase to levels that cause hypertension.
- Solution: If hematocrit increases it is a simple to reduce. Just give blood.
- The aromatase inhibitor inhibits testosterone to estrogen conversion via the aromatase enzyme. With low estrogen, bone loss can become an issue.
- Solution: Monitor estradiol and adjust the aromatase inhibitor so that estradiol is held in a low yet moderate range.
- There do not seem to be any proven mental issues from supraphysiological testosterone therapy. Some people feel more powerful and this can make them feel aggressive.
- Solution: Non-issue unless you have latent hostility issues.
- If an aromatase inhibitor is not used, then estrogen can increase dramatically and as a result gynecomastia can result (man boobs).
- Solution: Simply use an aromatase inhibitor to keep this from happening. If gyno becomes an issue make sure to measure your estriol and adjust your aromatase inhibitor as needed.
Requiring further research:
- One of the trials combined BAT with etoposide. Etoposide is derived from plant alkaloids and is a chemotherapy drug used to block DNA repair.
- What is the appropriate timing for prolactin reduction? Permanent? Intermittent? Temporary? Does it matter? (Cabergoline reduced my body fat from around 13% to 10% in about a month. I went off Cabergoline and it started going up, I quickly went back on and my body fat dropped)
- What about pregnenolone? By itself it looks to me like it might not be beneficial if you have PCa. The main drawback that I see is that it is a precursor for estrogen. Using an aromatase inhibitor should remove that negative. But with my hormonal program it might help. The estriol component of estrogens is probably beneficial for PCa and, since I take Zytiga and it interferes with corticosteroids, possibly the corticosteroid increase might be beneficial for blood pressure and overall health). However, estrone is likely bad for PCa. If I add this in I will have a baseline estrone test done and then see how estrone reacts. It’s rather straightforward to keep estradiol in check so this one should not necessarily be a concern.
4. DHEA: similar thoughts as pregnenolone. 7-keto-DHEA might be safer, or at least less complicated, since it doesn’t convert into any sex hormones.
5. DHT seems like a double edged sword. When is it good? When is it bad? What should the level be when T is high and estriol is low? Should it be pulsed?
6. Genistein and Daidzein (soy isoflavones) preferentially bind to ERb. But do they activate ERb or do they prevent it’s activation since estrogens can’t activate it?
7. Does Zytiga add anything to the supraphysiological testosterone treatments? If it doesn’t perhaps it would still be needed if a low testosterone phase is anticipated. And if it isn’t needed during the supraphysiological testosterone phase, perhaps it shouldn’t be used so that PCa cells don’t become desensitized to Zytiga (a suspected effect and when they aren’t sensitive to Zytiga an effective treatment is removed from the table).
Please note that this type of treatment works on some men but not on others. There is much speculation as to why but, as far as I know, nothing has been proven. So I feel that it is mandatory to have a baseline PSA (and scans depending on one’s situation) and monitor with additional testing.
Good site for education/research: https://www.endotext.org/section/male/
Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It’s Time to Sound the Alarm – PubMed
https://pubmed.ncbi.nlm.nih.gov/32202088/
Comparison of Testosterone Replacement Therapy Medications in the Treatment of Hypogonadism
https://www.npjournal.org/article/S1555-4155(16)30716-4/pdf
Testosterone Therapy in Men with Advanced Prostate Cancer
https://grandroundsinurology.com/testosterone-therapy-in-men-with-advanced-prostate-cancer/
2020 Prostate Cancer Highlight – High dose testosterone causes DNA damage and suppresses prostate cancer growth , Prostate Cancer Research Program, Congressionally Directed Medical Research Programs
https://cdmrp.army.mil/pcrp/research_highlights/20schweizer_highlight
HSD3B1 Genotypes Conferring Adrenal-Restrictive and Adrenal-Permissive Phenotypes in Prostate Cancer and Beyond | Endocrinology | Oxford Academic
https://academic.oup.com/endo/article/160/9/2180/5527766
Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and steroid reductase (SRD5A2) | Carcinogenesis | Oxford Academic
https://academic.oup.com/carcin/article/20/9/1727/261591
Evaluation of androgen antagonism of estrogen effect by dihydrotestosterone – ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/0022473183911287
Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5742814/
CRS0010040293.TIF
https://cancerres.aacrjournals.org/content/canres/1/4/293.full.pdf
Current opinion on the role of testosterone in the development of prostate cancer: a dynamic model | BMC Cancer | Full Text
https://bmccancer.biomedcentral.com/articles/10.1186/s12885-015-1833-5
Huggins’ research
https://cancerres.aacrjournals.org/content/canres/25/7_Part_1/1163.full.pdf
Testosterone slows prostate cancer recurrence in low-risk patients
https://medicalxpress.com/news/2019-03-testosterone-prostate-cancer-recurrence-low-risk.html
Supraphysiological androgens suppress prostate cancer growth through androgen receptor–mediated DNA damage
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6763228/
Frontiers | Supraphysiological Testosterone Therapy as Treatment for Castration-Resistant Prostate Cancer | Oncology
https://www.frontiersin.org/articles/10.3389/fonc.2018.00167/full
Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway | Molecular Cancer | Full Text
https://molecular-cancer.biomedcentral.com/articles/10.1186/1476-4598-13-214
Testosterone therapy in men with prostate cancer: literature review, clinical experience, and recommendations Morgentaler A, Conners WP – Asian J Androl
https://www.ajandrology.com/article.asp?issn=1008-682X;year=2015;volume=17;issue=2;spage=206;epage=211;aulast=Morgentaler
Testosterone Therapy in Men with Advanced Prostate Cancer – YouTube
https://www.youtube.com/watch?v=wafNZV-Hkqk
Cancers | Free Full-Text | Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions | HTML
https://www.mdpi.com/2072-6694/9/12/166/htm
Testosterone metabolites inhibit proliferation of castration- and therapy-resistant prostate cancer – PubMed
https://pubmed.ncbi.nlm.nih.gov/29682196/
Biphasic Effect of Androgens on Prostate Cancer Cells and Its Correlation With Androgen Receptor Coactivator Dopa Decarboxylase – Shao – 2007 – Journal of Andrology – Wiley Online Library
https://onlinelibrary.wiley.com/doi/pdf/10.2164/jandrol.106.002154
Raising Testosterone Naturally – Time Machine Hormone Rejuvenation
http://www.lifekick.us/raising-testosterone-naturally/
Durable Response of Enzalutamide-resistant Prostate Cancer to Supraphysiological Testosterone Is Associated with a Multifaceted Growth Suppression and Impaired DNA Damage Response Transcriptomic Program in Patient-derived Xenografts – PubMed
https://pubmed.ncbi.nlm.nih.gov/31227306/
Testosterone Therapy in Men With Localized Prostate Cancer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2312346/
Cancers | Free Full-Text | Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions
https://www.mdpi.com/2072-6694/9/12/166
Differential effects of 11 years of long-term injectable testosterone undecanoate therapy on anthropometric and metabolic parameters in hypogonadal men with normal weight, overweight and obesity in comparison with untreated controls: real-world data from a controlled registry study | International Journal of Obesity
https://www.nature.com/articles/s41366-019-0517-7
Does TRT really delay biochemical recurrence after radical prostatectomy (in selected patients)? | THE “NEW” PROSTATE CANCER INFOLINK
https://prostatecancerinfolink.net/2019/04/13/does-trt-really-delay-biochemical-recurrence-after-radical-prostatectomy-in-selected-patients/
Circulating steroid hormone variations throughout different stages of prostate cancer in: Endocrine-Related Cancer Volume 24 Issue 11 (2017)
https://erc.bioscientifica.com/view/journals/erc/24/11/ERC-17-0155.xml
The Palpable Prostate: Testosterone Metabolism and Prostate Cancer
https://palpable-prostate.blogspot.com/2008/02/testosterone-metabolism-and-prostate.html
Edward Friedman’s home page
http://www.math.uchicago.edu/~ed/
For Castrate-Resistant Prostate Cancer: High-Dose Testosterone
https://www.hopkinsmedicine.org/brady-urology-institute/patient-information/books-publications/articles/for-castrate-resistant-prostate-cancer-high-dose-testosterone
Boosting Testosterone Not Shown to Raise Prostate Cancer Risk
https://www.hopkinsmedicine.org/brady-urology-institute/patient-information/books-publications/articles/boosting-testosterone-not-shown-to-raise-prostate-cancer-risk
Testosterone as a Drug
https://www.hopkinsmedicine.org/news/articles/testosterone-as-a-drug
Do statins lower testosterone and does it matter?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3621773/
Prostate Cancer News, Reviews & Views: Testosterone to TREAT prostate cancer – are they crazy? No – it just may work. (mCRPC)
https://www.prostatecancer.news/2016/09/testosterone-to-treat-prostate-cancer.html
Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU145 prostate cancer cells | Molecular Cancer | Full Text
https://molecular-cancer.biomedcentral.com/articles/10.1186/1476-4598-7-88?fbclid=IwAR0IL13r5mcHDJxpcOZ5vvMBaMYrWpeGu-qQx7s16r–_5gENjpisb95tbs
Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5742814/
Prostate Cancer News, Reviews & Views: testosterone
https://www.prostatecancer.news/search/label/testosterone
Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca 2+ -related mechanisms | Scientific Reports
https://www.nature.com/articles/srep13856
Just another perspective: A Calcium perspective – Cancer Treatments – from Research to Application
https://www.cancertreatmentsresearch.com/just-another-perspective-a-calcium-perspective/
Ca2+ oscillations induced by testosterone enhance neurite outgrowth | Journal of Cell Science
https://jcs.biologists.org/content/119/4/733
Overturning Dogma — Using Testosterone in Prostate Cancer
https://www.medscape.com/viewarticle/910647
The Palpable Prostate: Testosterone Metabolism and Prostate Cancer
https://palpable-prostate.blogspot.com/2008/02/testosterone-metabolism-and-prostate.html
Endocrinology of Male Reproduction Archives – Endotext
https://www.endotext.org/section/male/
Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage – PubMed
https://pubmed.ncbi.nlm.nih.gov/31310591/
Testosterone Therapy in Men with Advanced Prostate Cancer
https://grandroundsinurology.com/testosterone-therapy-in-men-with-advanced-prostate-cancer/
Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5742814/
Testosterone modulates cardiac contraction and calcium homeostasis: cellular and molecular mechanisms
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4411792/
Testosterone Induces an Intracellular Calcium Increase by a Nongenomic Mechanism in Cultured Rat Cardiac Myocytes | Endocrinology | Oxford Academic
https://academic.oup.com/endo/article/147/3/1386/2501184
Calcium, cancer and killing: The role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells – ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0167488912003485#:~:text=DAG%2C%20Ca2%20%2B%20and%20Ca,immune%20cells%20in%20cancer%20tissue.
Role of Calcium Signaling in Prostate Cancer Progression: Effects on Cancer Hallmarks and Bone Metastatic Mechanisms
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7281772/
The post-treatment testosterone debate | THE “NEW” PROSTATE CANCER INFOLINK
https://prostatecancerinfolink.net/2019/03/21/the-post-treatment-testosterone-debate/
Testosterone and prostate cancer risk: the plot thickens as study reveals intriguing clues | Prostate Cancer UK
https://prostatecanceruk.org/about-us/news-and-views/2017/11/testosterone-and-prostate-cancer-risk-the-plot-thickens
Preoperative low serum testosterone is associated with high-grade prostate cancer and an increased Gleason score upgrading – PubMed
https://pubmed.ncbi.nlm.nih.gov/26439747/
Low serum testosterone is a predictor of high-grade disease in patients with prostate cancer
https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302017000800704
Does testosterone cause prostate cancer? — The Nutrition Clinic
https://christinasantini.com/blog/2019/9/16/does-testosterone-cause-prostate-cancer
Prostate Cancer News, Reviews & Views: Testosterone Therapy Does Not Increase the Risks of Prostate Cancer Recurrence or Death After Definitive Treatment for Localized Disease
https://www.prostatecancer.news/2020/06/testosterone-therapy-does-not-increase.html
Prostate Cancer News, Reviews & Views: Testosterone to TREAT prostate cancer – are they crazy? No – it just may work. (mHSPC)
https://www.prostatecancer.news/2016/09/testosterone-to-treat-prostate-cancer_5.html
